What is CTMS?
Clinical Trial Management Systems (CTMS) are specialized software platforms that streamline the management of clinical trials across pharmaceutical and clinical research organizations. These platforms centralize planning, execution, and reporting activities. They support core functions such as contact management, timeline tracking, milestone oversight, and resource coordination. Therefore, when someone asks what CTMS in modern research operations is, the answer is simple: it serves as a secure, centralized, web-based enterprise system that supports the complex demands of clinical research programs.
Purpose of Clinical Trial Management Systems (CTMS)
Streamlining Clinical Trial Processes
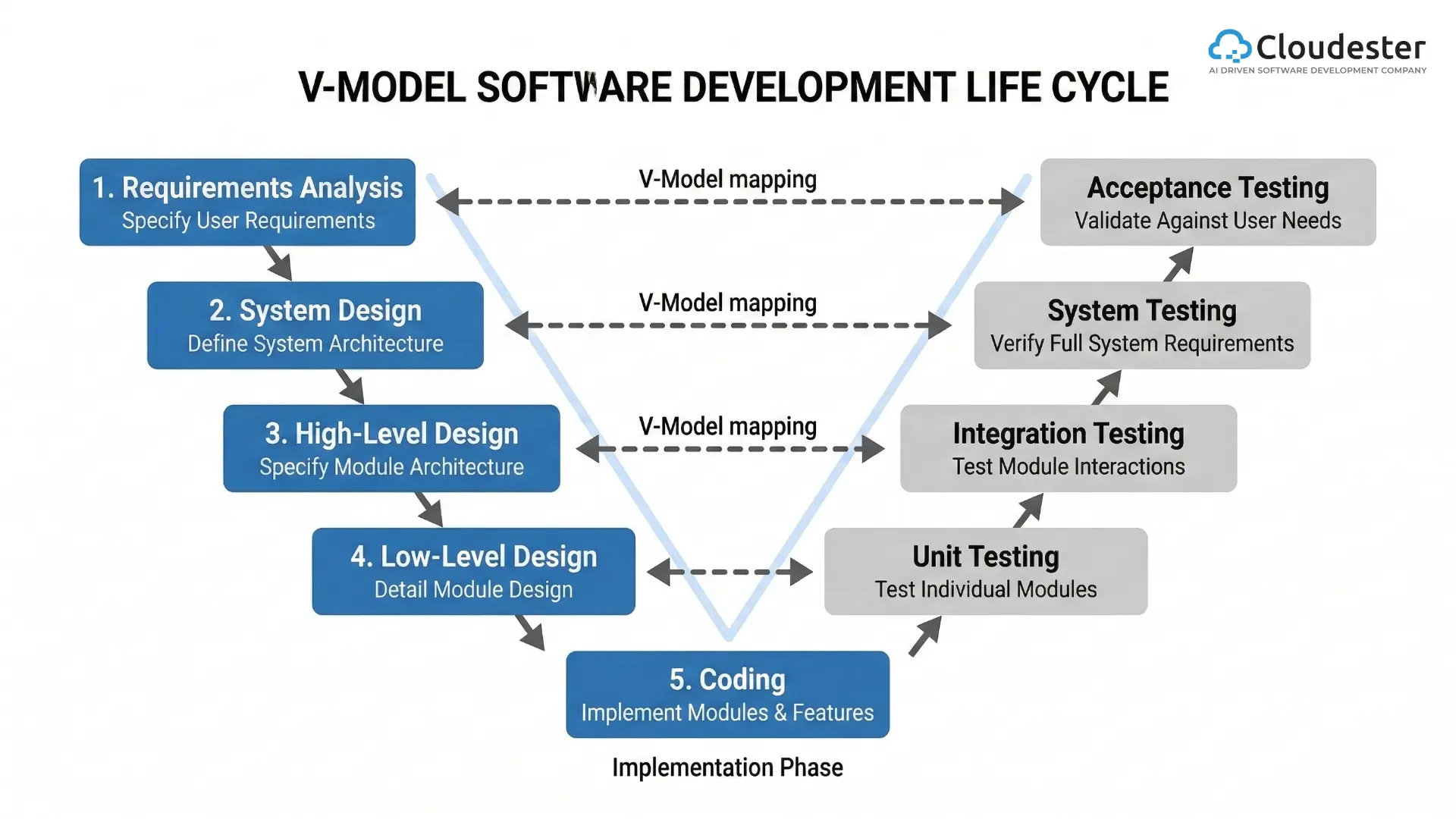
The primary purpose of CTMS is to help research teams plan, track, and control trial activities from startup through closeout. Early-stage teams sometimes manage data with spreadsheets and email. However, as studies scale and data volume increases, this approach becomes inefficient and risky. A CTMS offers structured workflows for compliance, subject tracking, documentation, budgeting, and reporting. It also connects with third party research systems to support integrated operations.
Enhancing Data Management
A CTMS creates a secure and centralized data repository for patient information, study documentation, and operational records. In addition, it provides a searchable database that allows users to locate information quickly, generate study reports, and share insights with internal and external stakeholders. This unified structure minimizes data duplication and improves audit readiness.
Custom AI Software Development Solution For Enterprises
Optimizing Financial Management
Beyond data control, a CTMS supports financial oversight. It enables accurate site payments, sponsor invoicing, and budget tracking. Teams can build study calendars, calculate costs, and ensure proper revenue recognition. As a result, financial transparency improves across the entire clinical trial lifecycle.
Boosting Overall Efficiency
CTMS platforms increase operational efficiency by reducing administrative tasks and preventing duplicate data entry. With a centralized system, staff retrieve trial information from one location and maintain consistent workflows. This allows study personnel to focus on scientific and operational objectives rather than manual tracking tasks.
Compliance Considerations for CTMS
Validation
CTMS validation includes structured documentation, test evidence, and change management. Each update requires tracking through a formal change log to maintain compliance and traceability.
Audit Trail
A CTMS maintains audit trails that track data changes, identify users making updates, and record reasons for modifications. This functionality supports transparency and regulatory oversight.
Access Control
System access must remain limited to trained and authorized individuals. Access logs should document user onboarding and removal timelines to support security and data protection.
Electronic Signatures
A compliant CTMS supports electronic signatures for approval workflows and regulatory documentation. This replaces physical signatures, improves speed, and maintains authenticity.
Security and Privacy
CTMS platforms must align with HIPAA and 21 CFR Part 11 requirements to protect clinical and patient data. HIPAA governs protected health information, and Part 11 governs electronic record integrity.
Penalties for Non-Compliance
- Tier 1: Unaware of violation: USD 100 to USD 50,000 per violation
- Tier 2: Aware but unable to avoid violation: USD 1,000 to USD 50,000
- Tier 3: Willful neglect with later correction: USD 10,000 to USD 50,000
- Tier 4: Willful neglect with no correction attempt: USD 50,000
Cloud Based CTMS
Modern CTMS platforms frequently operate using a SaaS model. They provide real time updates, secure remote access, data scalability, and lower infrastructure costs. This approach supports organizations of all sizes and allows flexible deployment across distributed research teams.
Cloudesters Expertise in e CTMS Solutions
Cloudester Software delivers advanced electronic CTMS platforms designed for pharmaceutical and clinical research organizations. Our e CTMS systems integrate with existing infrastructure, support real time tracking, and automate reporting. We focus on regulatory alignment with HIPAA and 21 CFR Part 11 while maintaining scalability and ease of use.
Teams manage patient recruitment, site oversight, and study closeout from one secure interface. With more than ten years of software development experience, we provide efficient workflows and support research organizations seeking a modern answer to what is CTMS and how it improves trial management.